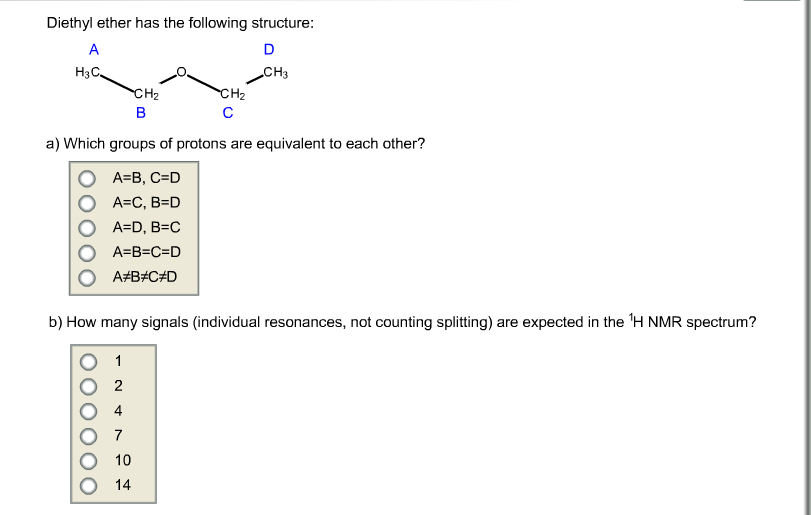
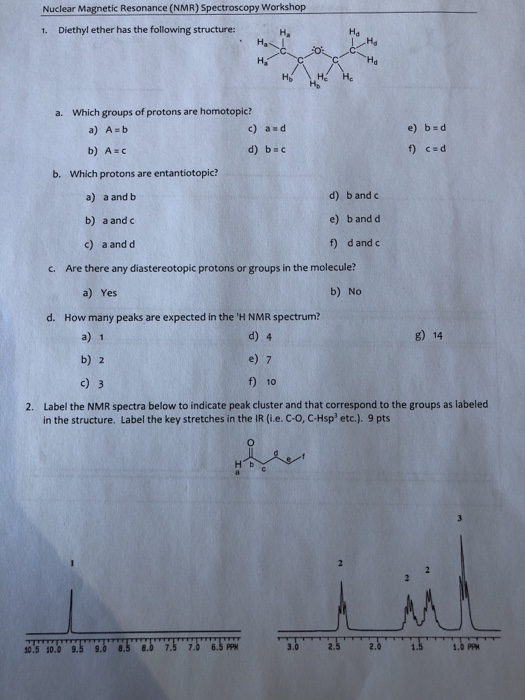
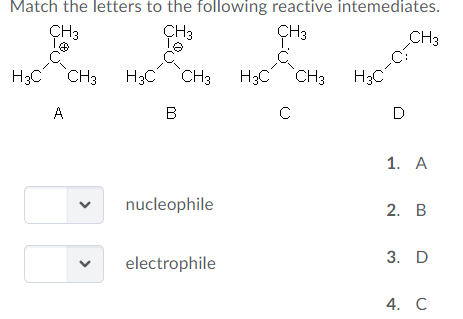
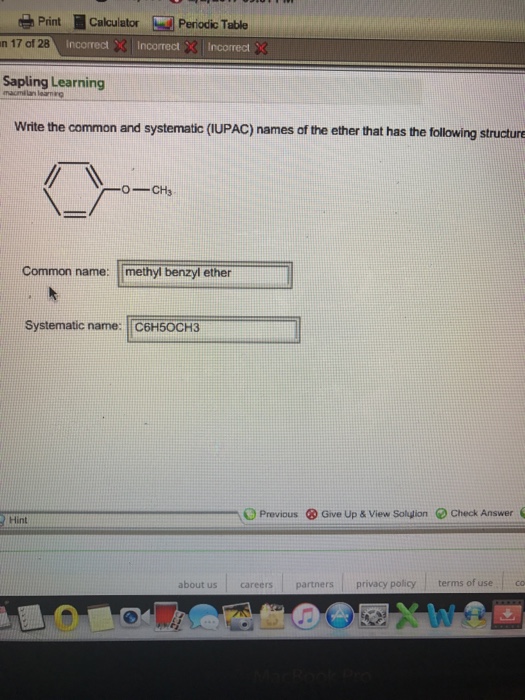
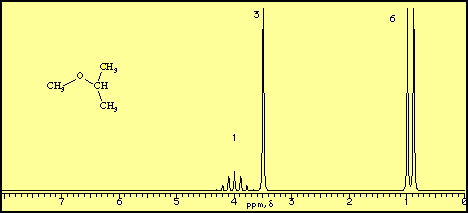
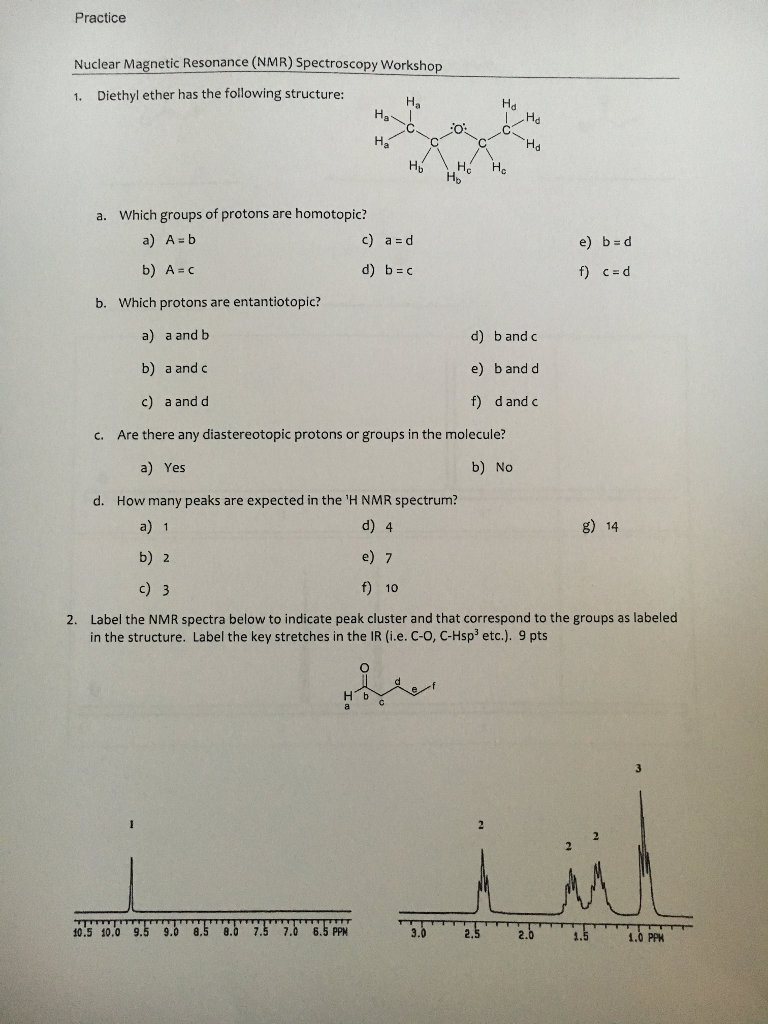
Diethyl Ether Has The Following Structure
So in pure dimethyl ether hydrogen bond does not occur.
Diethyl ether has the following structure. It is commonly used as a solvent in laboratories and as a starting fluid for some engines. What class of compound has the following general formula o r c nh2. Its molecular structure consists of two ethyl groups connected by an oxygen atom as in c2h5oc2h5. Diethyl ether has the following structure.
The structure of the dimethyl ether is as follows. Ethyl suggests that at least one of the functional groups which is bonded to the oxygen is well ethyl c 2 h 5 di ethyl reveals that not just one but two of the functional groups are in fact ethyl radicals. C methyl ethyl ether which of the following compounds is most soluble in water a ch3 ch2. How many signals individual resonances not counting splitting are expected in the 1h nmr spectrum.
Diethyl ether e none of the above. Get more help from chegg. Give the structure that corresponds to the following molecular formula and 1h nmr spectrum. What happens if you inhale diethyl ether.
Based on the information so far the structural formula is. It is a colorless highly volatile sweet smelling ethereal odour flammable liquid. Here no hydrogen atom is attached to oxygen atom. What class of compound has the following general formula o r c o r a ester b carboxylic acid c ketone d aldehyde e phenol.
Diethyl ether has the following structure which groups of protons are equivalent to each other. Ether suggests that you are looking at a compound with the general formula r 1 o r 2. Label each proton with the predicted splitting pattern it would exhibit in a 1h nmr spectrum. Ethyl ether also known as diethyl ether well known anesthetic generally referred to as simply ether an organic compound belonging to a large group of compounds called ether.
C 4 h 10 o.